Life span of worker honeybees reared in colonies kept on small-cell combs*)
KRZYSZTOF OLSZEWSKI, GRZEGORZ BORSUK, JERZY PALEOLOG, ANETA STRACHECKA
Department of Biological Bases of Animal Production, Faculty of Biology and Animal Breeding, University of Life Sciences in Lublin, Akademicka 13, 20-950 Lublin, Poland
Summary
Between 2011 and 2013, in laboratory cage tests, we compared life spans of bees reared in colonies kept on small-cell combs (cell width of 4.93 mm) that were either treated or untreated against varroatosis, as well as life spans of bees reared in colonies kept on standard-cell combs (cell width of 5.56 mm), both treated and untreated against varroatosis. Maintaining colonies on small-cell combs, combined with the lack of strong parasitic pressure from V. destructor, extended the life span of these bees in comparison with that of bees from standard-cell combs.The keeping of colonies on small-cell combs increased the longevity of bees reared on them and heavily infested by the parasites. Intensive infestation by V. destructor mites shortened the life span of bees, regardless of comb cell width, as confirmed by significant correlation coefficients between the parameters defining the scale of infestation and the life span of bees, while the specifics of the interrelation between the worker life span and the scale of infestation depended on the cell width (small/standard). Hence, comb cell width affects the biology of both the parasite and the host, as well as the relationship between them. Therefore, analysing biological connections between A. mellifera colonies and V. destructor in the context of different comb cell widths seems a very promising direction for research.
Bee comb cells in the nests of bees raised in Europe today are wider than in original, natural nests. This results from the invention of the comb foundation and the introduction of the frame hive with a dismount- able nest at the turn of the 19th century (9), associated with the enlargement and standardisation of comb cell width. The currently applied, so-called standard, comb cell width (in standard cells) ranges from 5.40 mm to 5.50 mm (7, 9), whereas the width of bee comb cells in natural nests of the Northern European dark bee ranged from 4.90 mm to 5.10 mm (Cowan 1904 following 9). The latter cells are defined as small cells (9). To date, little attention has been devoted to explain- ing how the change in cell width affected biologically important traits of bee colonies, which had developed over thousands of years of evolution.
Worker life spans are of key significance for such functional traits as the strength and survival rate of colonies. Increased life spans can be accompanied by growing honey yields, since the longer bees live,the more nectar foraging flights they can perform (3, 8, 25). On the other hand (8), short worker life spans can improve the health of the colony, as the compensation in the form of a quick generational replacement prevents diseases. Fluri (4) reports that the worker life span is affected in 80% by environmental conditions, the remaining 20% being determined by hereditary factors. This is in line with calculations by Rinderer et al. (17), who estimated the heritability coefficient for this trait at h2 = 0.23. This shows that studies of the influence of comb cell width, as an intra-colony environmental factor (23, 24), on the bee life span are particularly important.
Laboratory cage tests have been used to assess important bee traits for over 30 years (10). They make it possible to analyse exactly the genetic background of various traits through standardisation and a partial elimination of the influence of changeable environmental conditions. Such tests have been used to assess the efficiency of food intake and storage, worker life spans (2, 10, 11, 12, 14, 15), and intergenotypic worker interactions connected with the hoarding behavior (1).
Laboratory tests have also been applied to investigate the effect of pesticides (26), drugs and food supplements on bees (16, 21, 22).
In this study, by means of laboratory cage tests, we examined the life spans of bees reared in small- and standard-cell combs, both in (highly infested) colonies treated and untreated against varroatosis for the last three years. We assumed that the untreated colonies were under a strong selection pressure from V. destructor mites.
Material and methods
The life spans of worker bees reared in standard-cell comb colonies (cell width 5.56 mm) and small-cell comb colonies (cell width 4.93 mm) kept at the University of Life Sciences in Lublin (51°13’N, 22°38’E) were determined in laboratory cage tests.
In 2011, we compared the life spans of bees reared in colonies kept on small-cell combs and untreated against varroatosis (SM-UT) with the life spans of bees from colonies kept on standard-cell combs. treated (ST-T) and untreated (ST-UT) against varroatosis. The ST-T colonies were treated with Biowar 500, applying 2 strips of 500 mg Amitraz in each strip per colony from the beginning of August until the end of October. The SM-UT group consisted of 5 colonies. These were survivors from 16 colonies that had been transferred onto small-cell combs as early as 2008, and at the same time ceased to be treated against varroatosis. In the absence of such treatment, these colonies underwent natural selection (selection intensity of 5/16). The ST-T group comprised 10 colonies and the ST-UT group was made up of 5 colonies. The treatment of the ST-UT colonies was discontinued in 2009. The queens in the ST-T and ST-UT colonies were naturally mated sisters and descended from a queen representing a varroatosis untreated colony. The SM-UT queens descended from the same baseline population as ST-T and ST-UT, though from an untreated colony.
In 2012, we compared the life spans of bees reared in 10 SM-UT and 10 ST-T colonies formed of nuclei created in 2011. The SM-UT nuclei were provided with naturally mated daughter-queens reared from larvae drafted from two SM-UT colonies that had been the fastest to develop in spring and produced the largest amounts of honey. It was assumed that these two colonies were best adapted to sub- sisting on small-cell combs. The ST-T queens were sisters descended from the same mother queen as the ST-T queens in 2011. The same procedure as with the SM-UT and ST-T colonies, was applied to the ST-UT colonies. None of the ST-UT colonies survived the 2011/2012 overwintering.
In 2013, we compared the life spans of the bees in the SM-T and ST-T colonies to determine the effect of comb cell width on the longevity of bees in the colonies treated against varroatosis. Both of these groups comprised 10 colonies formed in 2012 on the basis of nuclei provided with natu- rally mated sister queens raised from larvae that had been drafted from a colony treated with an anti-varroatosis agent.
Each year, one-day-old worker bees from each colony of each group were collected using screen comb-cages, and after 24 hours introduced into 2 wooden cages, each containing 40 bees per colony, according to the methodology of Strachecka et al. (22). Next, every other day, dead bees were removed from each cage and counted. Bee life span distribution quartiles were adopted as a measure of apian longevity. It was determined on the basis of the number of days elapsed from the beginning of the test until the day on which 75%, 50% and 25% workers survived. In the colo- nies from which the one-day-old workers were collected, we determined how many brood cells were infested with V. destructor in May and June of 2011 and 2012. In May, we counted mature V. destructor females (brown cuticle hue) in the debris obtained with bottom-board drawers (5,20) and computed their mean numbers per 24 h.
The significance of the differences between the mean parameter values in SM-UT, ST-UT and ST-T colonies in 2011 was determined with Tukey’s test (SAS Institute Version 9.13., 2002-2003 license 86636). Worker life span means and rates of infestation by V. destructor in the SM-UT and ST-T colonies in 2012 and worker life span means in the SM-T and ST-T colonies in 2013 were compared using one-way ANOVA. Spearman coefficients of rank correlation were calculated between the mean worker life span and the number of cells containing infested brood, and between the mean life span and the number of mature V. destructor females fallen into debris during 24 hours.
Results and discussion
In 2011, the scale of infestation in the SM-UT and ST-UT colonies by V. destructor mites was similar, and higher than in the ST-T colonies (Tab. 1). A similar pattern was observed in 2012 when comparing infestation rates in the SM-UT and ST-T colonies.
In the SM-UT colonies, the life span of the bees was negatively correlated with the scale of infestation of the brood by V. destructor, whereas in the ST-UT colonies it was negatively correlated with the number of mature V. destructor mites fallen into debris during 24 hours (Tab. 2). A similar correlation as in the case of the ST-UT colonies was observed in the ST-T colonies, probably because these colonies were the least infested as a result of varroatosis treatment (Tab. 1).
Tab. 1. Infestation of colonies by V. destructor in 2011 and 2012
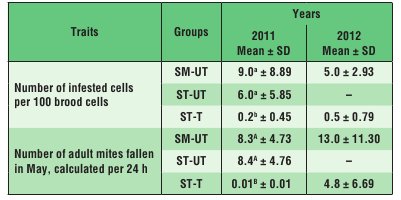
Explanations: SM-UT – untreated small-cell colonies; ST-UT
– untreated standard-cell colonies; ST-T – treated standard-cell colonies; SD – standard deviation; a, b – the differences within a given trait in a given year are significant at p ≤ 0.05; A, B – the differences within a given trait in a given quartile are significant at p ≤ 0.01.
Tab. 2. Spearman rank correlation coefficients computed for the worker life span and characteristics of colony infestation by V. destructor in years 2011 and 2012
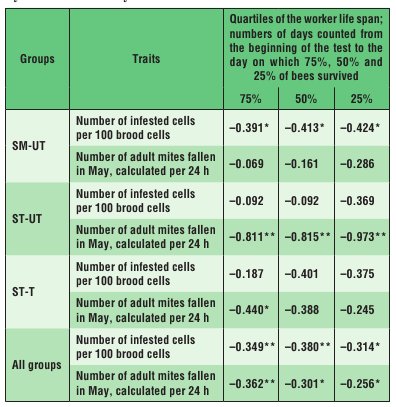
Explanations: SM-UT – untreated small-cell colonies; ST-UT – untreated standard-cell colonies; ST-T – treated standard-cell colonies; * the correlation is significant at p ≤ 0.05; ** the cor-relation is significant at p ≤ 0.01.
In 2013, the bees reared in the SM-T colonies lived longer than those in the ST-T ones (Fig. 1). The positive effect of keeping colonies on small-cell combs on the longevity of bees reared on them was confirmed by the results in 2012, when the SM-UT bees lived as long as the ST-T ones (Fig. 1). Only in 2011 did the bees raised in the SM-UT colonies live shorter than those in the ST-T colonies, while, except for the life span of 75% of the bees, they did not differ from those in the ST-UT colonies.
Longer life spans of the bees from the SM-T colonies in comparison with the ST-T colonies show that, in the absence of a strong pressure from V. destructor (Fig. 1, year 2013), the keeping of colonies on small-cell combs increases the longevity of bees reared on them. This is confirmed by the results for 2012, when the bees from the SM-UT colonies, exposed to a strong parasite pressure, lived as long as those from the treated colonies (ST-T). We make this assumption, since De Jong and De Jong (6) and Schneider and Drescher (19) demonstrated that increased bee colony infestation by V. destructor shortens worker life spans.
Our yet unpublished observations, compared with the results of this paper, lead to the conclusion that the longer life spans of bees from the SM-T colonies, determined in laboratory cage tests (Fig. 1, year 2013), corresponded with a greater strength of those colonies during the overwintering period and in early spring, in comparison with the ST-T colonies. The exact identification of the connections between the life span, the keeping of bees on small-cell combs and colony strength requires further studies. Apart from cell width, the apian life span is, most probably, also significantly affected by other intra-colony habitat factors (4, 17).
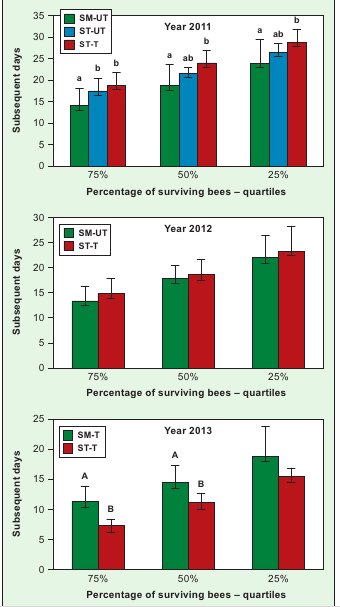
Fig. 1. Quartiles of the worker life span in laboratory cage testsExplanations: SM-UT – bees from untreated small-cell colonies; SM-T – bees from treated small-cell colonies; ST-UT – bees from untreated standard-cell colonies; ST-T – bees from treated standard-cell colonies; a, b – the differences between groups within a given quartile are significant at p ≤ 0.05; A, B – the dif-ferences between groups within a given quartile are significant at p ≤ 0.01; Vertical bars indicate standard deviation.
Only in 2011 did the bees reared in the SM-UT colonies live shorter than those in the ST-T ones (Fig. 1). It is intriguing that 75% of the bees reared in the ST- -UT colonies lived longer than the bees reared in the SM-UT colonies, despite the fact that both groups did not differ in the scale of infestation by V. destructor (Fig. 1, Tab. 1).
As expected (4, 19), strong infestation of the colonies by V. destructor mites reduced the apian life span, regardless of the cell width, as confirmed by significant negative coefficients of correlation between the parameters defining the scale of infestation and the life span (Tab. 1). The life-span reduction was greater in bees reared on small combs. The fact that, despite a similar scale of infestation of the SM-UT and ST-UT colonies by V. destructor (Tab. 1), the life span of bees reared in the SM-UT colonies was negatively correlated with the brood infestation rate, whereas the life span of the ST-UT bees was negatively correlated with the number of the parasite females fallen into debris within 24 hours (Tab. 2), suggests that the character of correlation between the worker life span and the infestation rate in colonies kept on small-cell combs is different from that in colonies kept on large-cell combs. Hence, the comb cell width affects the biology of both the parasite and the host, as well as the relationship between them (13).The worker life span can be significantly influenced both by the comb cell width (small or standard), which is regarded as an internal factor of the bee nest habi-tat (23, 24), and by the pressure from V. destructor. The rearing of workers on small-cell combs seems to lengthen their lifespan. Therefore, analysing biological connections between A. mellifera colonies and V. de-structor mites in the context of different combs cell widths seems a very promising direction for further research.
References
1. Borsuk G., Olszewski K., Strachecka A., Paleolog J.: The interaction of worker bees which have increased genotype variance, Part 2. cage tests of sugar syrup collecting and mortality. J. Apic. Sci. 2011, 55(1), 59-65.
2. Bruckner D.: Hoarding behaviour and life span of inbred, non-inbred and hybrid honeybees. J. Apic. Res. 1980, 19(1), 35-41.
3. Bruder A.: Meine Betriebsweise. Ehrenwirt Verlag, München 1983.
4. Fluri P.: Zur Regulation der Lebensdauer der Bienen. Deut. Bienen J. 1993, 1(11), 4-7.
5. Howis M., Nowakowski P.: Varroa destructor removal efficiency using Beevital hive clean preparation. J. Apic. Sci. 2009, 53(2), 17-27.
6. Jong D. de, De Jong P. H.: Longevity of Africanized Honey Bees (Hymeno- ptera: Apidae) Infested by Varroa jacobsoni (Parasitiformes: Varroidae). J. Econ. Entomol. 1983, 76(4), 766-768.
7. Kober T.: Zurück zur kleinen Biene, Teil 1: Die Geschichte der Zellgrößen. Imkerfreund 2003, 58(4), 8-10.
8. Liebig G.: Über das Lebensalter der Bienen. Deut. Bienen J. 2002, 10(2), 4-6.
9. McMullan J. B., Brown M. J. F.: The influence of small-cell brood combs on the morphometry of honeybees (Apis mellifera). Apidologie 2006, 37, 665-672.
10. Milne Ch. P.: An Improved Laboratory Measurement of Hoarding Behaviour in the Honey Bee. Amer. Bee J. 1977, 117(8), 502-507.
11. Milne Ch. P.: Laboratory measurement of honey production in the honeybee. 1. A model for hoarding behaviour by caged workers. J. Apic. Res. 1980, 19(2), 122-126.
12. Milne Ch. P.: The need for using laboratory tests in breeding honeybees for improved honey production. J. Apic. Res. 1985, 24(4), 237-242.
13. Olszewski K.: Biologia, użytkowość oraz oporność na Varroa destructor rodzin pszczelich utrzymywanych na plastrach o małych komórkach. Wydawnictwo Uniwersytetu Przyrodniczego w Lublinie, Rozprawa habilitacyjna 2013, z. 372, ISSN 1899-2374.
14. Olszewski K., Paleolog J.: Foraging and hoarding efficiency in Buckfast purebreds and Norwegian Black Bee (A. m. mellifera) hybrids Part 1. Annual honey yield versus results of field flying cage and laboratory tests. J. Apic. Sci. 2005, 49(1), 45-53.
15. Paleolog J., Olszewski K.: Foraging and hoarding efficiency in Buckfast pure-breds and Norwegian Black Bee (A. m. mellifera) hybrids Part 2. Comparison with the Caucasian bee hybrids under flying cage and laboratory test conditions. J. Apic. Sci. 2005, 49(1), 67-79.
16. Paleolog J., Strachecka A., Burzyński S., Olszewski K., Borsuk G.: The larval diet supplemented with the low-molecular epigenetic switch sodium phenyl- acetylglutaminate influences the worker cuticle proteolytic system in Apis mellifera L. 2011, 55(2), 73-83.
17. Rinderer T. E., Collins A. M., Brown M. A.: Heritabilities and correlations of the honey bee: response to Nosema apis, longevity, and alarm response to isopentyl acetate. Apidologie 1983, 14, 79-85.
18. Rinderer T. E., Danka R. G., Stelzer J. A.: Seasonal inconsistencies in the relationship between honey bee longevity in field colonies and laboratory cages. J. Apic. Res. 2012, 51(2), 218-219.
19. Schneider P., Drescher W.: Einfluss der Parasitierung durch die Milbe Varroa jacobsoni Oud. auf das Schlupfgewicht, die Gewichtsentwicklung, die Entwicklung der Popharynxdrüsen und die Lebensdauer von Apis mellifera L. Apidologie 1987, 18, 101-110.
20. Semkiw P., Skubida P., Pohorecka K.: The amitraz strips efficacy in control of Varroa destructor after many years application of amitraz in apiaries. J. Apic. Sci. 2013, 57(1), 17-27.
21. Strachecka A., Borsuk G., Olszewski K., Gagoś M., Gryzińska M., Paleolog J., Chobotow J., Nawrocka A.: The effect of amphotericin b on mortality, protein concentrations and DNA methylation level in the honey bee (Apis mellifera). J. Apic. Sci. 2012, 56(2), 107-113.
22. Strachecka A., Olszewski K., Paleolog J., Borsuk G., Bajda M.: Coenzyme Q10 treatments influence the lifespan and key biochemical resistance systems in the honeybee, Apis mellifera. Archives of Insect Biochemistry and Physiology 2014, 86, 1-15.
23. Tautz J.: Phänomen Honigbienen. Elsevier GmbH, Spektrum Akademischer Verlag, München 2007.
24. Winston M. L.: The Biology of the Honey Bee. Harvard University Press Cambridge, Massachusetts London 1987.
25. Woyke J.: Correlations and interactions between population, length of worker life and honey production by honeybees in temperate region. J. Apicult. Res. 1984, 23(3), 148-154.
26. Wu J. Y., Anelli C. M., Sheppard W. S.: Sub-Lethal Effects of Pesticide Residues in Brood Comb on Worker Honey Bee (Apis mellifera) Development and Longevity PLoS ONE. 2011, 6(2), 1-11.
Corresponding author: Krzysztof Olszewski PhD, ul. Akademicka 13, 20-950 Lublin;
*) This study was supported by National Science Centre grant No. N N311542140 in 2011-2014.
